Spaser as a biological probe
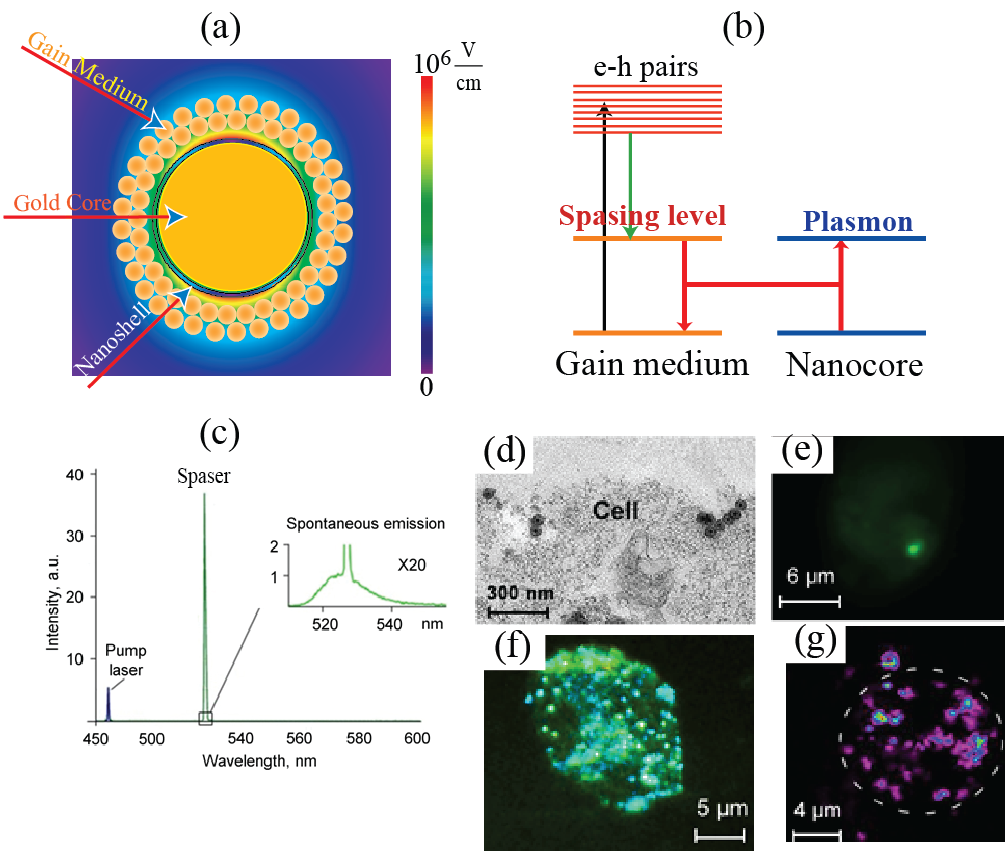
To diagnose and treat cancer, it is of primary importance to be able to see and differentiate them from normal tissues. For this purpose, one highlights the cancer cells by marking them with labels that usually are fluorescent objects: dye molecules or tiny semiconductor nanospheres called quantum dots. A requirement for such labels is that they emit bright light under optical illumination. This emission should be bright enough and have characteristic colors to be distinguishable from light scattering produced by the living tissues1. For use in vivo, these labels should be biocompatible and non-toxic for a human organism. Some of the labels can also be used for therapy, in particular, for photodynamic therapy2 or for thermal therapy3: killing the cancer cells due to photo-production of chemically active singlet oxygen or heat. Dye molecules or semiconductor colloidal quantum dots (SCQD’s) as fluorescent labels have common fundamental drawback: their emitting intensity is limited by the radiative decay rate of the fluorescing excite state (usually on the order of a nanosecond) and saturates (levels off) when the laser intensity increases to a moderate level on the order of a MW/cm2. This intensity can potentially be increased by increasing the concentration of the fluorescent label but the toxicity may become problematic in such a case, especially for the SCQD’s. Additionally, the absorption and emission lines of organic dyes are spectrally rather wide, which limits the achievable spectral selectivity. This disadvantage of wide spectra and low spectral selectivity is especially pronounced for the plasmonic nanoparticles used as theranostic agents3. A radically novel idea is pioneered in a recent article4 by Galanzha et al. where theranostic applications of a spaser (Surface Plasmon Amplification by Stimulated Emission of Radiation) are introduced and developed. The spaser as a phenomenon and device was proposed in 2003 by Bergman and Stockman5-6 and later developed and observed in a large number of works, in particular, see selected citations in ref. 4. Spaser is a plasmonic counterpart of laser where photons (quanta of electromagnetic field) are replaced by surface plasmons (quanta of electromechanical oscillations). The cavity (resonator) of a laser is replaced in the spaser by a metal nanoparticle (the spaser core), which supports surface plasmons whose fields are tightly nanolocalized in the vicinity of this core. Excitation of the spaser with an external laser pump causes generation of electron-hole pairs in the spaser shell and the stimulated near-field emission of coherent plasmons into the metal nanoparticle core (“resonator”) of the spaser – see Fig. 1 (a) and (b). Today the spasers are observed in a wide spectral range, from ultraviolet, across all visible, and into mid infrared; they are of many configuration and sizes. Now it looks like there will always be a spaser suitable for any particular nanoscopic job.
The fundamental advantage of the spaser over the conventional laser in micro- and nanoscopic application is that it is a near-field quantum generator, and its size can be much smaller than the wavelength – on the order of sizes of biological molecules and viruses. Its fundamental advantage also is that in contrast to common fluorescent labels its emission does not saturate: it is a stimulated emission and its intensity linearly depends on the pump power as long as the spaser is not physically damaged. Consequently, the brightness of the spaser and the energy released inside the targeted cell are orders of magnitude greater for the spaser than for conventional labels. The intensity and spectral brightness of the spaser radiating inside a living cell are of unprecedented total and, especially, spectral brightness – see Fig. 1 (c). A new intriguing property of transient vapor nanobubble around spaser that it can play the role of dynamic optical resonator with positive feedback amplifying spaser light and creating thus “giant” lasing that is important for cancer diagnosis at cellular levels in deep tissue. The spasers are chemically conjugated with folate to selectively target tumor cells internalized them as seen in Fig. 1 (d). The radiation of the spaser is so bright that even one spaser is well seen inside the cell [Fig. 1 (e)]. Multiple spasers produce unprecedented bright images within a cell [Fig. 1 (f)]. They are also excellent agents for photothermal and photoacoustic imaging because they also are equally efficient, unsaturable nano-generators of heat inside the cells – see [Fig. 1 (g)], which are greatly advantageous over conventional imaging and theranostics agents7-9. Moreover, ultrasharp photoacoustic resonances and red-blue resonance spitting8 can improve identification of targeted cancer cells in complex absorption background. The enormously large and unsaturable optical absorption by the spasers and the corresponding efficient generation of heat, nanobubbles8 and shock waves inside the cell allows one to effectively use them for theranostics – just a few laser pulses are sufficient to reliably kill tumor cells using photomechanical effects within the irradiated volume without damaging the healthy cells. Indeed, laser-induced nanobubbles around the heated spaser during fast expansion and collapse provide the high kinetic energy and localized pressure that can damage vital structures of triple negative breast cancer cells that are resistant to conventional therapy. One can envision that it will become possible to detect and eliminate single CTC’s as they pass through a blood vessels: a very intense and highly monochromatic (narrow-spectral-band) radiation of the spasers will allow one to continuously monitor the passage of the CTC’s through surface blood vessels. As soon as a CTC is detected via its spaser radiation, a high-power laser pulse is sent that kills these CTC’s, one tumor cell at a time. And this only the beginning of using the spaser phenomenon in biomedicine. References
|
|||





 Cancer tumors are known for their ability to proliferate or metastasize. This happens either through cancer cells spreading through lymphatic nodes or by the so called circulating tumor cells (CTC’s). In the difficult task of cancer diagnostics and therapeutics (the so-called theranostics), the easiest task may be removal or destruction of the primary tumor but fighting the cancer cells migrating through the lymphatic system at the CTC’s traveling through blood vessels is an altogether different and much more difficult problem.
Cancer tumors are known for their ability to proliferate or metastasize. This happens either through cancer cells spreading through lymphatic nodes or by the so called circulating tumor cells (CTC’s). In the difficult task of cancer diagnostics and therapeutics (the so-called theranostics), the easiest task may be removal or destruction of the primary tumor but fighting the cancer cells migrating through the lymphatic system at the CTC’s traveling through blood vessels is an altogether different and much more difficult problem.